From affinity selection to kinetic selection in Germinal Centre modelling
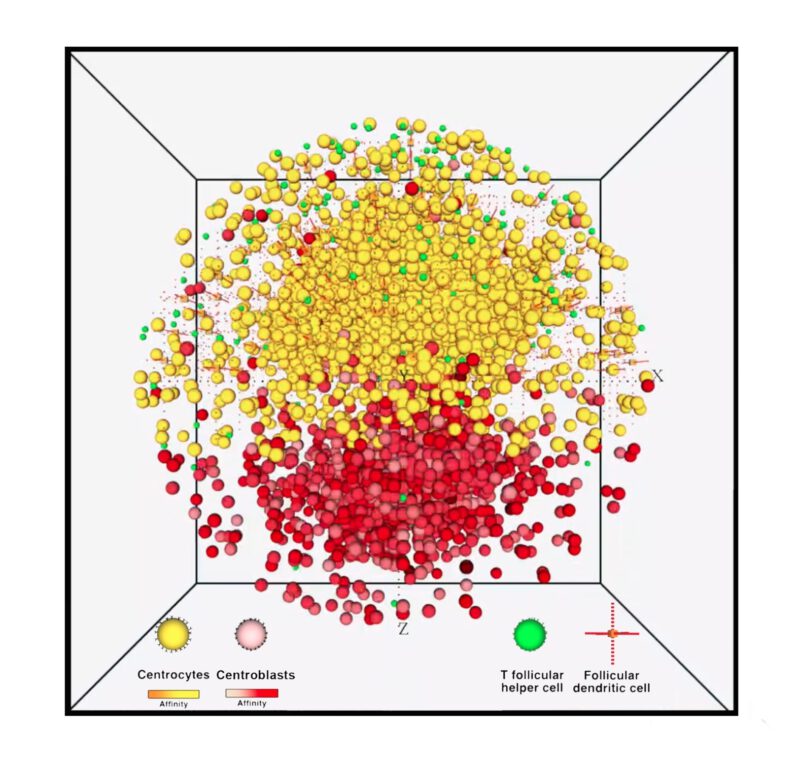
An illustration of a simulated GC reaction in silico. Credit:DL
Recently our work on kinetics maturation of B cells in Germinal centres was published in the journal of PLOS computational biology.
Adaptive immunity is one of the vital defence mechanisms of the human body to fight virtually unlimited types of pathogens by producing antigen-specific high-affinity antibodies that bind to pathogens and neutralise them or mark them for further elimination. Affinity is a quantity used to measure and report the strength of interaction between antibodies and antigens that depends both on how fast antibodies bind to antigens (association rate) and how long the bond lasts (dissociation rate). The affinity of produced antibodies for a specific antigen increases in germinal centres through a process called affinity maturation, during which B cells with higher affinities have a competitive advantage and get positively selected to differentiate into antibody-producing plasma cells. Our research shows that the mechanism by which B cells capture Ag affects GC dynamics and GC output with respect to B-cell receptor kinetics. Notably, in a mechanism where rupture of CC-FDC bonds is possible during Ag extraction, B-cell clones with low dissociation rates outcompete clones with high association rates over time. Understanding how B cells get selected in germinal centres could help to develop an optimised and effective immune response against a disease through vaccination for a fast-operating and long-lasting immune response. Read more here.





