3TR
3TR aims to provide fundamental new insights into the mechanisms of response and non-response to treatment. Thereby, we challenge the conventional “single disease”-based approach, in which diseases are classified according to their end-organ involvement rather than the molecular pathways underpinning them.
Consequently, we propose a paradigm shift in the way we look at diseases: Promoting a scientific evidence-based rationale for treatment selection, rather than following the traditional trial and error approach, 3TR will have significant implications for future patient management and the assessment of efficacy, safety and quality of future health products.
Aldo Jongejan works at the Bioinformatics Laboratory on the 3TR project. The 3TR project is coordinated by Prof. Marta E. Alarcón-Riquelme GENYO centre at the Fundación Pública Andaluza Progreso y Salud (FPS)

For more information: https://3tr-imi.eu/
3TR Scientific Aims
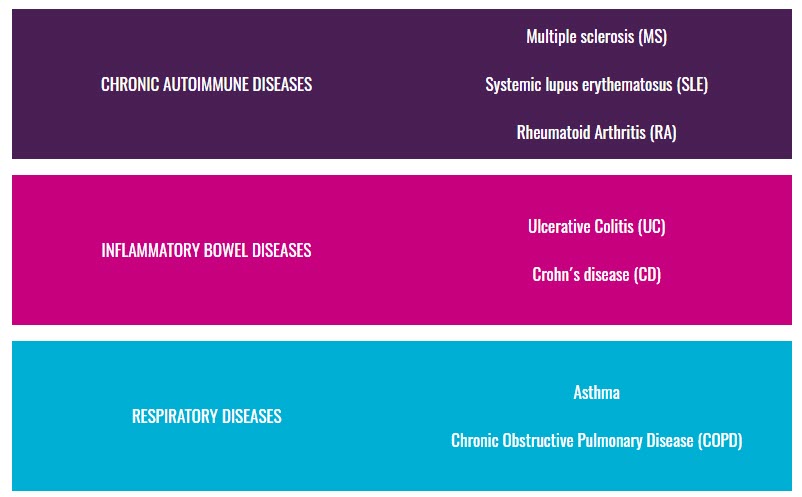
Autoimmune, inflammatory and allergic diseases are highly heterogeneous conditions in their clinical phenotype. However, despite their heterogeneity, it has been shown that they share genetic risk, share some disease pathways, and share specific clinical manifestations.
Consequently, individuals with a disease may share an inflammatory molecular pattern with individuals with the other diseases. Hence, they may also share pathways of response to treatment and disease progression, which we will further investigate.
3TR will integrate the analysis of seven autoimmune, allergic, and inflammatory conditions to identify the relationship between longitudinal molecular and microbiome profiles in blood cells and tissues, and disease trajectories to characterise and better predict, why certain patient groups do not respond to certain treatments.
The research of dr. Aldo Jongejan focuses on the bioinformatics analysis of single cell RNAseq data and the reconstruction and modelling of gene networks in the context of systemic lupus erythematosus (SLE). In particular we will focus on lupus nephritis. This project is a collaboration with prof. dr. Ronald van Vollenhoven, prof. dr. Alexandre Voskuyl, and prof. dr. Sergey Nejentsev.


Innovative Medicines Initiative
H2020