Plasma cell differentation in the germinal center
Sponsor: Amsterdam UMC
Project leaders
Prof.dr. Antoine H.C. van Kampen
Bioinformatics Laboratory, Amsterdam UMC
Department of pathology, Lymphoma and Myeloma Center Amsterdam (LYMMCARE), Amsterdam UMC
Biosystems Data Analysis, SILS, University of Amsterdam
Introduction
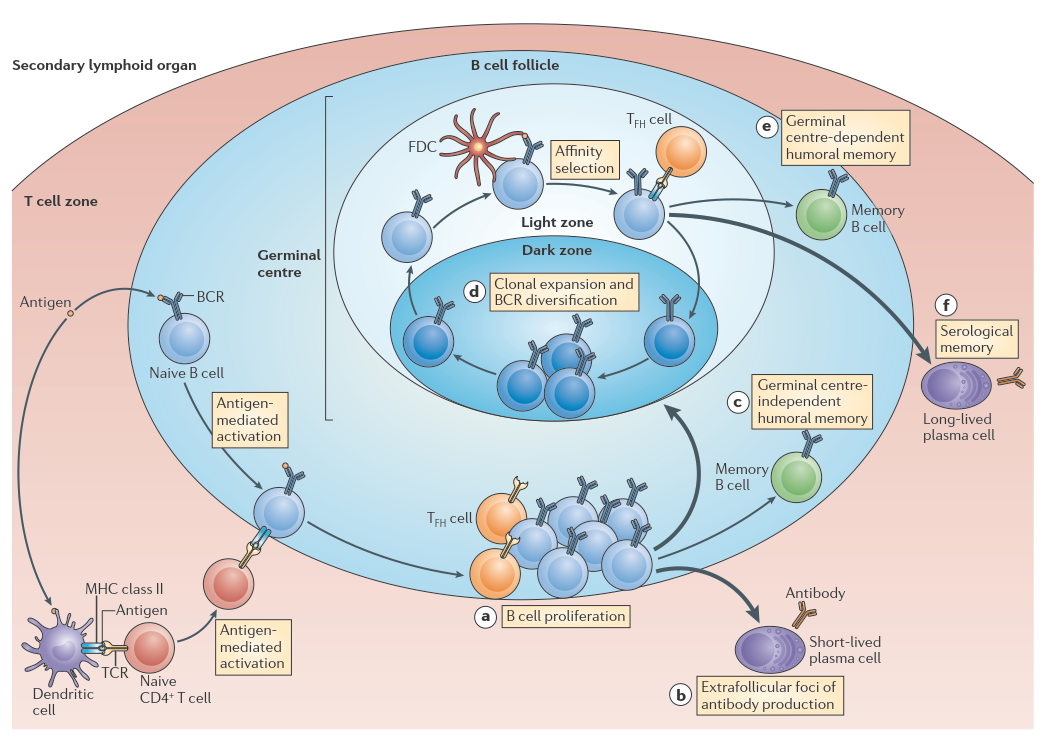
Germinal centers (GCs; Figure 1; Kurosaki, 2015; Victora, 2014) are anatomical structures located inside B-cell follicles within secondary lymphoid organs that play an important role in the adaptive immune system. Through subsequent rounds of cell proliferation, somatic hypermutation (SHM) and positive selection the B-cell receptor (BcR) is optimized for antigen (Ag) binding in a process called affinity maturation. This eventually results in the development of memory B-cells (MBCs) and plasma cells (PCs) that produce high affinity antibodies (Abs), which provide an effective immune protection. GCs comprise two functional zones. In the dark zone (DZ) centroblasts (CBs) rapidly proliferate and accumulate SHMs in the genes that encode their BcR. The light zone (LZ) is mainly characterized by the presence of centrocytes (CCs), follicular dendritic cells (FDCs) that present Ag in the form of immune complexes to GC B cells, and T follicular helper (Tfh) cells. CCs capture and internalize Ag through their BcR in an affinity-dependent manner triggering survival signals. Subsequently, the Ag is processed by the CCs resulting in class II MHC-peptide complexes (pMHCII) presented to the Tfh cells. Hence, B cells compete in an affinity-dependent way for interaction with Tfh cells, facilitating CD40 and cytokine signaling to become positively selected. Positively selected CCs may return to the CB state and recycle to the DZ to undergo further rounds of proliferation and SHM. Alternatively, positively selected CCs may differentiate to MBCs or PCs. Recently, it was also shown that GC B-cell migration influences PC development. The cellular and molecular mechanisms that regulate PC and MBC differentiation remain largely unknown, while such knowledge would crucially advance our understanding of GC-associated diseases such as B-cell lymphomas and autoimmune disorders.
Figure 1. Germinal center reaction. Figure copied from Kurosaki (2015).
Overall aim
We aim to develop a multiscale model that integrates molecular and cellular mechanisms involved in GC plasma cell differentation.
Method and Approach
Key-obective 1. Development of a multiscale model to study plasma cell differentation
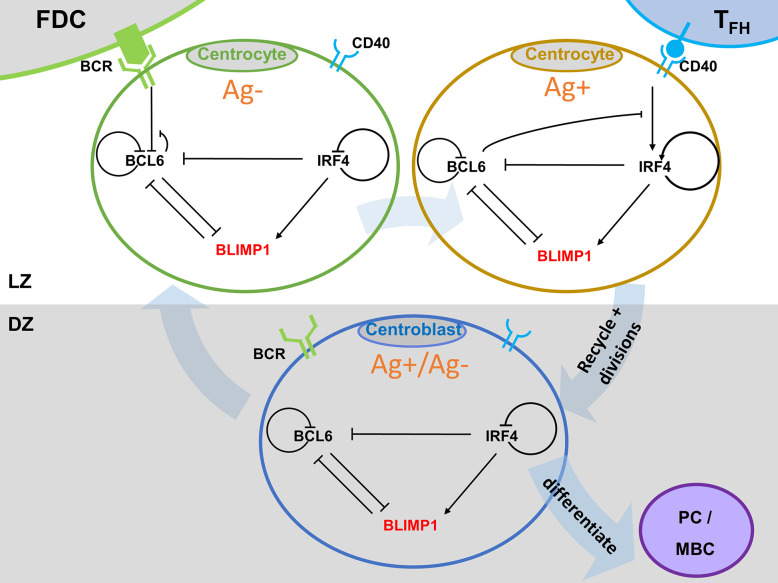
GCs play a key role in the adaptive immune system since they produce terminally differentiated memory B cells (MBC) and plasma cells (PCs) which produce high affinity antibodies for an effective immune protection. From a mechanistic perspective, gene regulatory networks (GRN) including a many different transcription factors (TF) have been found to play key roles in cellular processes that take place during the GC reaction and influence B-cell fate. Vice versa, cellular interactions and B-cell division may affect the status of the GRNs. Multiscale computational models (MSM) can be used to describe and integrate the different scales of biological systems in order to gain understanding of their spatiotemporal dynamics and the interaction between the scales. We will implement a multiscale model (Figure 2) to study PC differentation. We will integrate an Agent-based models (ABM) model and a ordinary differential equation based model of a core GRN. The ABM will be used to simulate the temporal and spatial GC dynamics and outcome in different scenarios for PC differentation. We will extend and modify a pre-existing ABM of the GC developed by prof. dr. Michael Meyer-Hermann (Meyer-Hermann, 2012). This model implements the iterative process of affinity maturation: cell proliferation, somatic hypermutation (SHM), and positive selection of high affinity B-cells. This results in an increase of B-cell receptor (BcR) affinity and the production of high affinity plasma cells. A video of a GC simulation with the ABM was made by Danial Lashgari. The GRN will be based on a model from dr. Maria Martinez (Martinez, 2012).
Figure 2. Multiscale model for PC differentiation. The cellular model (ABM) and molecular GRN (ODE) models are integrated by embedding the GRN in each B-cell and output cell of the ABM. Signals through the BcR (FDC interaction) and CD40 (Tfh interaction) change the state (TF concentrations) of the GRN which is updated at every time step of the ABM. A positively selected CC becomes Ag+ by definition.
Key-objective 2. Investigate the role of affinity-based T follicular help (Tfh) cell help.
We will use the multiscale model to investigate the putative role of asymmetric B-cell division during PC differentiation. We propose that asymmetric division of Ag and TFs might contribute to PC differentiation.
Key-objective 3. Investigate the effect of genetic alterations in the context of DLBCL
We will investigate different scenarios of genetic alterations leading to deregulation of the core BCL6-IRF4-BLIMP1 GRN used in our multiscale model, to investigate GC dynamics in Diffuse large B-cell lymphoma (DLBCL).
References
- Kurosaki T, Kometani K, Ise W. 2015. Nat Rev Immunol 15: 149-59
- Meyer-Hermann M, Mohr E, Pelletier N, Zhang Y, Victora GD, Toellner KM. 2012. Cell Rep 2: 162-74
- Martínez MR, Corradin A, Klein U, Álvarez MJ, Toffolo GM, di Camillo B, Califano A, Stolovitzky GA. 2012. Proc Natl Acad Sci U S A 109(7): 2672-7